


DUAL GLUCAGON AGONIST; GLP-1 AGONIST
ACTIVE SUBSTANCE: 10 MG SURVODUTIDE
FORM: 2 ML VIAL
ACTIVE HALF-LIFE: 109-115 HOURS
DOSAGE: MEN 0.6-4.8 MG/WEEK
ACNE: PERHAPS
WATER RETENTION: NO
HBR: NO
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: DRAGON PHARMA, EUROPE
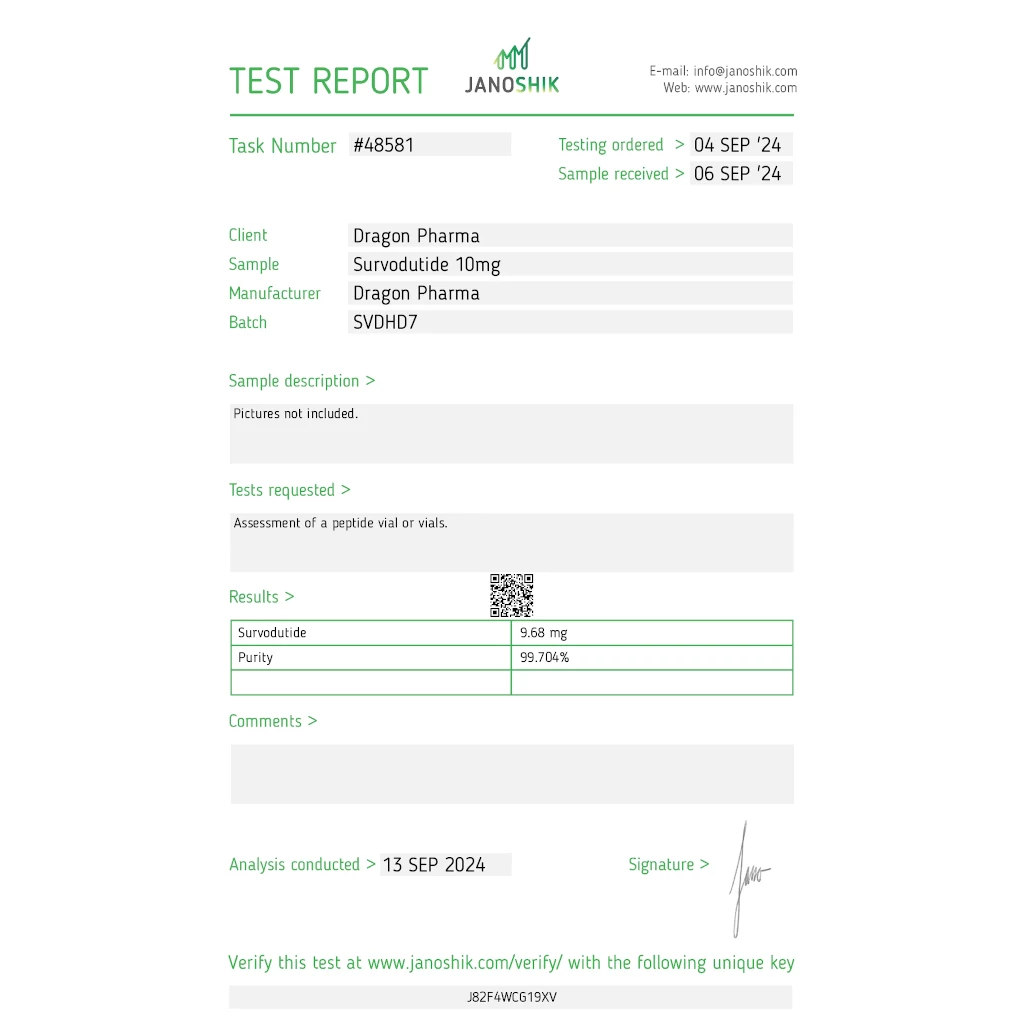
LABORATORY TESTED: VIEW LAB RESULTS
DragonPharma.net online store sells anabolic steroids, we have only original peptides, so you can buy from us original Survodutide 10mg.

Survodutide is a cutting-edge pharmaceutical compound made by Dragon Pharma, designed to aid in weight loss and metabolic regulation. Each 2 mL vial contains 10 mg of the active substance Survodutide, a dual agonist that targets both glucagon and GLP-1 receptors, playing a key role in controlling appetite and increasing energy expenditure. By enhancing metabolic activity and reducing hunger, Survodutide is highly effective for individuals struggling with obesity, insulin resistance, or metabolic disorders.
Survodutide is typically administered through subcutaneous injection, once per week. The treatment often starts with a low dose to allow the body to adjust, followed by gradual increases to the maintenance dose over several weeks. The recommended dosage is determined by the individual's needs, but cycles generally include an escalation phase of up to 20 weeks, after which the maintenance phase continues at the optimum dosage, which is typically between 3.6 mg and 4.8 mg per week.
This gradual escalation helps minimize potential side effects, and it is important that users follow the dosage instructions carefully. Survodutide is a potent compound, and its effectiveness can be maximized when used alongside dietary modifications and regular physical activity.
An example Survodutide cycle could begin with an initial dose of 0.6 mg per week for the first 4 weeks, followed by incremental increases to 2.4 mg by week 8, 3.6 mg by week 12, and reaching the maximum recommended dose of 4.8 mg by week 16. The user would then maintain the 4.8 mg dosage for an additional 10 to 16 weeks, depending on the individual's progress and tolerance.
Throughout the cycle, regular monitoring by a healthcare professional is essential to ensure that the body is responding positively to the treatment. Adjustments to the cycle may be made based on individual results.
While Dragon Pharma Survodutide is generally well-tolerated, some users may experience side effects, particularly during the initial phase of treatment. The most common side effects include gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal discomfort. These side effects tend to diminish as the body adapts to the treatment.
Other potential side effects include fatigue, dizziness, and headaches. In rare cases, more serious adverse effects such as heart-related issues or kidney complications may occur, particularly in individuals with pre-existing health conditions. It's crucial for users to have their kidney and heart functions monitored throughout the cycle, especially if using Survodutide for an extended period.
Overall, Survodutide represents a significant advancement in weight loss and metabolic therapies, offering a powerful tool for individuals seeking to improve their health and well-being. When used under proper medical supervision, it can help achieve significant results in body composition, appetite control, and metabolic efficiency.
Please log in to write SURVODUTIDE 10 MG review.

CLASSIFICATION: GLP-1 RECEPTOR AGONIST
ACTIVE SUBSTANCE: SEMAGLUTIDE
FORM: LYOPHILIZED POWDER – 2 ML VIAL (5 MG/VIAL)
ACTIVE HALF-LIFE: ~7 DAYS
DOSAGE: 0.25 - 0.5 MG/WEEK
ACNE: NO
WATER RETENTION: NO
HIGH BLOOD PRESSURE (HBR): NO
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: DRAGON PHARMA, EUROPE
LABORATORY TESTED: VIEW LAB RESULTS
CLASSIFICATION: GIP/GLP-1/GLUCAGON RECEPTOR TRIAGONIST
ACTIVE SUBSTANCE: RETATRUTIDE
FORM: 2 ML VIAL x 10 MG
ACTIVE HALF-LIFE: ~ 6 DAYS
DOSAGE: 0.5 MG/WEEK
ACNE: N/A
WATER RETENTION: N/A
HBR: N/A
HEPATOTOXICITY: N/A
AROMATIZATION: N/A
MANUFACTURER: DRAGON PHARMA
LABORATORY TESTED: VIEW LAB RESULTS
CLASSIFICATION: GLP-1 RECEPTOR AGONIST
ACTIVE SUBSTANCE: TIRZEPATIDE
FORM: LYOPHILIZED POWDER – 2 ML VIAL (5 MG/VIAL)
ACTIVE HALF-LIFE: ~5 DAYS
DOSAGE: 2.5 - 5 MG/WEEK (RESEARCH USE ONLY)
ACNE: NO
WATER RETENTION: NO
HIGH BLOOD PRESSURE (HBR): NO
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: DRAGON PHARMA, EUROPE
LABORATORY TESTED: VIEW LAB RESULTS
Nov 23, 2024 (10:11)
Legit stuff that delivers fast results